Introduction: High grade B-cell lymphoma with MYC, BCL2 and/or BCL6 rearrangements (HGBCL MYC/BCL2 and/or BCL6-R) is a group of aggressive lymphomas associated with poor outcomes when treated with R-CHOP. Most HGBCL present in advanced stage and have a high risk for CNS relapse. MYC partner (Immunoglobulin (IG) vs. Non-IG) status has been correlated with clinical outcomes. Recent data suggests that MYC/BCL6 translocations may not portend the same inferior outcomes as MYC/BCL2-R and that limited stage HGBCL MYC/BCL2-R may have similar PFS and OS when treated with intensive chemotherapy vs. R-CHOP. In this study, we aimed to build upon the paucity of data available by describing the clinical characteristics of limited stage HGBCL including the risk of CNS relapse, and the role of MYC partner on clinical outcome.
Methods: We performed a retrospective analysis of pts with newly diagnosed HGBCL MYC/BCL2 and/or BCL6-R at MSKCC from 1/2010 to 9/2022. All pts had pathological confirmation conducted at MSKCC. Patient demographics, disease/treatment history, clinical response (CRR and ORR), PFS and OS were extracted from electronic medical record. Stage modified IPI (smIPI) [age>60, elevated LDH, Stage II/IIE, ECOG ≥2] and Stage Adjusted IPI (stIPI) [age >60, elevated LDH, ECOG ≥2] were calculated. PFS and OS were estimated using the Kaplan-Meier method, and comparisons were made using log-rank test. Associations between survival outcomes, clinical, and treatment characteristics were evaluated using Cox regression models, from which HRs and corresponding 95% CIs were obtained. FISH for MYC partner was performed on archived tissue.
Results: A total of 188 patients were identified with HGBCL MYC/BCL2 and/or BCL6-R. Nine pts were excluded due to limited information, and 41 pts were identified as having limited stage disease. Median age at diagnosis was 60 years (22-88 years), 63% had Stage II disease, 34% had extranodal involvement of which 50% involved the GI tract, 65% had a smIPI of 0-1, 75% with stIPI of 0-1, and 24% had transformed disease. Regarding rearrangement status, 41% of patients had MYC/BCL2-R 22% were characterized by MYC/BCL6-R and 37% had MYC/BCL2/BCL6-R . The median diagnosis-to-treatment interval was 23 days. First-line treatment included DA-R-EPOCH (76%), R-CHOP (20%), R-CODOX-M-IVAC (2%), and R-GCVP (2%) (median of 6 cycles). Twenty-seven percent received radiation. Thirty-two percent of patients received CNS prophylaxis, with the majority receiving IT-chemotherapy alone (median of 4 doses).
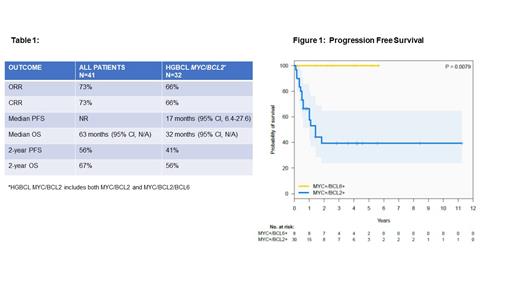
After a median follow-up of 48 months, the median PFS was not reached and median OS was 63 months, respectively (Table 1). The 2-year PFS and OS were 56% and 67%, respectively. The ORR and CRR were both 73%. Fifteen patients had relapse/refractory disease, with the majority being primary refractory (12/15). Seven pts received salvage with ASCT (n=1), CAR T-cell therapy (n=4) or both (n=2). Eleven died due to progressive disease. No CNS relapses occurred. MYC/BCL2-R had inferior outcomes with a median PFS of 17 months (95% CI 6.4-27.6) as compared to MYC/BCL6-R (not reached) (P=0.0079, Fig 1), and there was no additional impact on PFS and OS if MYC/BCL2/BCL6-R was present compared to MYC/BCL2-R alone. In a univariate analysis, elevated LDH (p=0.04), MYC/BCL2 (p=0.009), and stIPI ≥2 (p=0.0025) were associated with inferior PFS as well as OS. On the contrary, extranodal disease, age, ECOG ≥2, smIPI, bulky disease, consolidation with radiation, transformed disease, and intensive chemotherapy (DA-R-EPOCH) did not impact outcome. MYC partner evaluation and copy number analysis (n=31) is in progress.
Conclusions: In our series,pts with limited stage MYC/BCL2-R had poor outcomes while MYC/BCL6-R experienced favorable outcomes. There was no additional prognostic impact when BCL6-R is present with MYC/BCL2-R . This adds to the growing evidence that MYC/BCL6-R is not a poor prognostic factor in HGBCL. Primary refractory disease defines the majority of treatment failures in our study and is associated with dismal outcomes, although our CAR-T cell experience was limited due time period of this study. Despite this, our results emphasize the need for improved treatment strategies for this patient population. Ongoing analysis of MYC partner and copy number analysis may reveal genetic mechanisms driving outcomes. No CNS relapses occurred, suggesting that CNS prophylaxis can be safely omitted for the majority.
Disclosures
Lue:OncLive: Consultancy; Merck: Consultancy. Epstein-Peterson:WebMD: Honoraria; OncLive: Honoraria; Amgen: Research Funding; Viracta: Research Funding; Kymera: Research Funding. Falchi:Genentech: Consultancy, Other: Advisory Board, Research Funding; ADC Therapeutics: Other: Advisory Board; AstraZeneca: Consultancy; Seagen: Other: Advisory Board; Abbvie: Consultancy, Other: Advisory Board, Research Funding; Genmab: Consultancy, Research Funding; Roche: Consultancy, Research Funding. Ghione:AstraZeneca Pharmaceuticals: Consultancy; Kite, A Gilead Company: Research Funding; Kyowa Hakko Kirin: Consultancy; Secura Bio: Consultancy. Hamlin:ADC Therapeutics: Consultancy. Horwitz:SecuraBio: Consultancy; Crispr Therapeutics: Research Funding; Kyowa Hakko Kirin: Consultancy, Research Funding; Abcuro Inc.: Consultancy; Tubulis: Consultancy; Millenium: Research Funding; ADC Therapeutics: Research Funding; Yingli Pharma Limited: Consultancy; ONO Pharmaceuticals: Consultancy; Auxilius Pharma: Consultancy; Affimed: Research Funding; Trillium Therapeutics: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Shoreline Biosciences, Inc.: Consultancy; Cimieo Therapeutics: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Seattle Genetics: Research Funding; Verastem/SecuraBio: Research Funding; Celgene: Research Funding. Johnson:Myeloid Therapeutics: Consultancy. Kumar:Adaptive Biotechnologies: Research Funding; Pharmacyclics: Research Funding; Genentech: Consultancy, Research Funding; Loxo/Lily Oncology: Consultancy, Research Funding; Janssen: Consultancy; Kite Pharma: Consultancy; Beigene: Research Funding; BridgeBio: Current equity holder in publicly-traded company; Celgene: Research Funding; Seattle Genetics: Research Funding; Astra Zeneca: Consultancy, Research Funding; Abbvie Pharmaceuticals: Research Funding. Moskowitz:Beigene: Research Funding; Incyte: Research Funding; Seattle Genetics: Honoraria, Research Funding; Merck: Honoraria, Research Funding; Bristol-Myers Squibb: Research Funding; ADC Therapeutics: Research Funding. Palomba:BMS: Honoraria; Ceramedix: Honoraria; Juno: Honoraria, Patents & Royalties; Kite: Honoraria; MustangBio: Honoraria; Novartis: Honoraria; GarudaTherapeutics: Honoraria; Cellectar: Honoraria; Pluto Immunotherapeutics: Honoraria; Rheos: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; Smart Immune: Honoraria; Thymofox: Honoraria; Synthekine: Honoraria. Torka:ADC Therapeutics: Consultancy; Genentech: Consultancy; Genmab: Consultancy; Seagen: Consultancy; TG Therapeutics: Consultancy; Lilly USA: Consultancy. Vardhana:Immunai: Consultancy; Koch Disruptive Technologies: Consultancy. Zelenetz:AstraZeneca: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria, Research Funding; Lymphoma Research Foundation: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; SAB: Membership on an entity's Board of Directors or advisory committees; None other than mutual funds (401K): Current equity holder in publicly-traded company; Janssen Pharmaceuticals: Consultancy, Honoraria; Abbvie: Research Funding; Gilead: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; MEI Pharma Inc: Consultancy, Honoraria, Research Funding. Dogan:Seattle Genetics: Consultancy; Physicians' Education Resource: Consultancy, Honoraria; EUSA Pharma: Consultancy; Loxo: Consultancy; Peer View: Honoraria; Incyte: Consultancy; Takeda: Other: Research Funding; Roche: Other: Research Funding. Salles:Janssen: Consultancy, Research Funding; Merck: Consultancy, Honoraria; BeiGene: Consultancy; Orna: Consultancy; Ipsen: Consultancy, Research Funding; Nordic Nanovector: Consultancy; ATB Therapeutics: Consultancy; BMS/Celgene: Consultancy; Novartis: Consultancy; Nurix: Consultancy; AbbVie: Consultancy, Honoraria; Owkin: Current holder of stock options in a privately-held company; Incyte: Consultancy; Genmab: Consultancy; Molecular Partners: Consultancy; Debiopharm: Consultancy; EPIZYME: Consultancy; Loxo/Lilly: Consultancy; Kite/Gilead: Consultancy; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal